Translational Pulmonology
Scientific Background
Asthma is a common complex chronic inflammatory disease affecting up to 30% of children and adults in some regions of the world. Hallmark characteristics include reversible airway obstruction in the presence of airway inflammation and airway hyperresponsiveness (AHR); symptoms can range from mild to life threatening. Most of the 30 million people suffering from asthma are well treated due to improved therapeutic strategies. However, patients are far from being symptom-free with upwards of 10-20% labelled as severe or uncontrolled; cases of fatal asthma attacks are still not rare events.
One significant subset of asthma patients presents with features of both asthma and chronic obstructive pulmonary disease (COPD). COPD is currently rated the fourth most common cause of death globally and is predicted to be the third by 2030. The presence of asthma and COPD in the same patient affects about 10-25% of COPD patients. The clinical presentation of co-existing COPD and asthma leads to significantly worse respiratory symptoms, poorer quality of life, and increased risk of exacerbations and hospital admissions. However, the concept of co-existing COPD and asthma is controversial and no single accepted definition exists which raises an important question as to its origin and how to clinically classify and manage these patients remains a puzzle.
A co-existence of asthma has also been observed in approximately 19% of patients with cystic fibrosis (CF). CF is the most common, lethal genetic disease in Caucasian populations. It is difficult to determine which CF patients have concomitant asthma and which have asthma-like symptoms caused by CF lung inflammation as established and validated criteria are lacking due to the overlap of clinical measurements. Inhaled corticosteroids are the mainstay treatment of asthma and are some of the most commonly prescribed medications in CF. However, the widespread prescription of inhaled corticosteroids is not supported due to uncertain benefits on pulmonary function in CF patients, specifically in patients with concomitant CF and asthma.
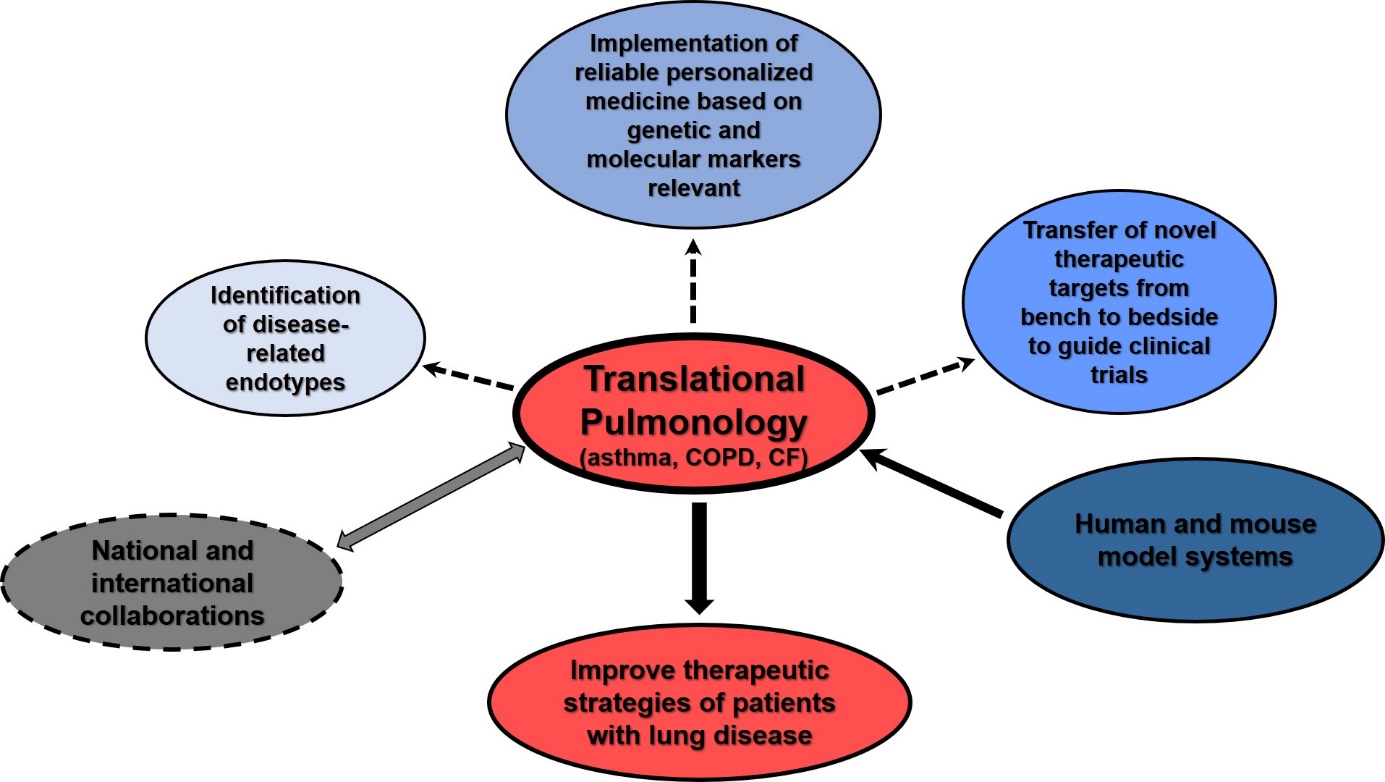
Clustering of patients with these complex lung diseases based on clinical and physiological parameters has had some benefit in understanding its heterogeneity. However, it is suggested that different mechanisms (endotypes) are responsible for the heterogeneity seen between patients with asthma, COPD, CF or in patients with multiple lung diseases. The implementation of functional genomic studies and other ‘omics technologies using human and mouse model systems has provided a powerful tool to identify novel risk factors and distinguish those markers truly promoting disease. Prof. Dr. Schedel has contributed with a number of novel concepts in the field following this approach with the overarching goal to characterize cellular and molecular markers to
- distinguish subsets of patients with lung disease(s) driven by different endotypes
- guide a more personalized medicine approach
- discover novel treatment strategies to improve patient care.
Research Areas
Molecular and functional principles of lung diseases
- Genomic predisposition to allergic respiratory disease, chronic obstructive pulmonary disease (COPD), and cystic fibrosis (CF)
- Genetic and environmental risk factors influencing the development, exacerbation, and treatment of respiratory diseases
- Identification of predictive biomarkers using molecular approaches
- Functional consequences of dysregulated biological pathways
- T cell subpopulations in lung diseases – function and regulation
- Airway epithelial cells and their role in respiratory disease
Identification of reliable markers to achieve personalized medicine
- Analyses of mouse model systems to test and manipulate causal pathways related to disease development and progression
- Human in vitro model systems to modulate immune responses
- Characterization of disease-related mechanisms in patients with lung disease
- Discovery of novel molecules driving disease
Evaluation of alternative treatment strategies in patients with respiratory diseases
- Examination of patient samples and clinical parameters before and after treatment
- Development and application of mouse models to analyze the function and efficacy of new treatment approaches
- Transfer of novel therapeutic targets from bench to bedside
Model Systems
In vitro models:
- Cell lines (Jurkat T cells, HEK293, A549, YT, NIH3T3)
- Primary cells (human and mouse CD4+ T cells, CD8+ T cells, nasal epithelial cells, airway epithelial cells)
In vivo models:
- Models of experimental asthma (allergen, viral, bacterial)
- Genetic or functional manipulation of disease-related pathways
Methods
- Cell Culture
- Cloning
- Genotyping of single nucleotide polymorphisms (SNPs)
- Electrophoretic Mobility Shift Assay (EMSA)
- Chromatin immunoprecipitation (ChIP)
- Luciferase Assay
- ELISA
- Gene and protein expression analyses
- Flow cytometry
- Histological and immunohistological methods
- Lung function analysis in mice
- Protein-protein interaction analyses (with collaborators)
- RNA sequencing (with collaborators)
- Computational genetic and genomic analyses
